BioMarin Pharmaceutical’s (BMRN) Phearless Phase 1/2 gene therapy study on adults with phenylketonuria has been put on hold by the U.S. Food and Drug Administration (FDA). The regulator's decision is based on interim safety findings from a pre-clinical, non-GLP pharmacology study.
BioMarin is a biotechnology company, which engages in the development and commercialization of therapies for people with serious and life-threatening rare diseases.
The pre-clinical study was performed on 63 animals of which seven were administered a high dose. Six of them developed tumors on liver necropsy 52 weeks after dosing. Additionally, five of these animals had adenomas and one had hepatocellular carcinoma (HCC).
Therefore, the company said, “The translatability of these findings to humans is uncertain and under further investigation.” (See BioMarin stock chart on TipRanks)
BioMarin has stopped taking further enrollment into the study until the investigation of these findings is completed. Furthermore, it is coordinating with the FDA and other health authorities and will communicate the next steps for the program when available.
Following the development, Piper Sandler analyst Christopher Raymond maintained a Buy rating on BioMarin with a price target of $119 (51.5% upside potential from current levels).
Raymond is of the opinion that with little certainty into timelines and the potential for impact to BioMarin's other gene therapy programs, the market is likely to assign a higher discount rate to these efforts going forward.
Consensus among analysts is a Strong Buy based on 7 Buys and 2 Holds. The average BioMarin price target stands at $113.38, which implies upside potential of 44.4%.
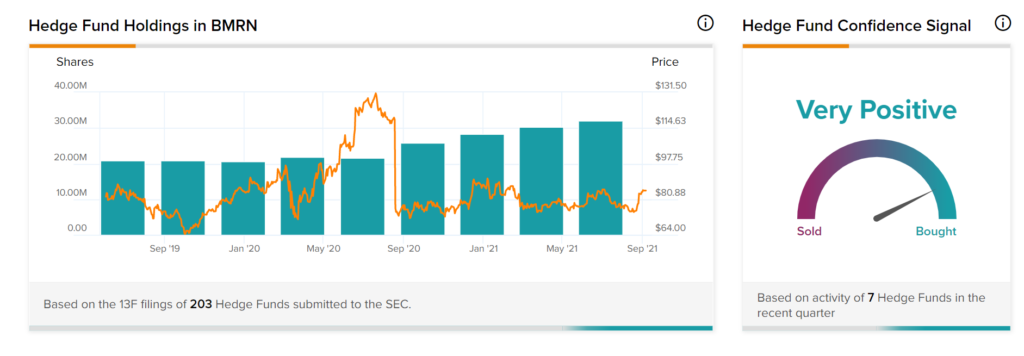
TipRanks’ Hedge Fund Trading Activity tool shows that confidence in BioMarin is currently Very Positive, as the cumulative change in holdings across all 7 hedge funds that were active in the last quarter was an increase of 1.8 million shares.
Related News:
Google, Others Seek to Meet Malaysian PM to Discuss Cabotage Policy
ICL Procures €250 Million Sustainability Linked Loan
VEON Announces Sale of Russian Tower Assets For $970M; Street Says Buy
The post FDA Places BioMarin’s Gene Therapy Study on Hold appeared first on TipRanks Financial Blog.
----------------------------
By: Radhika Saraogi
Title: FDA Places BioMarin’s Gene Therapy Study on Hold
Sourced From: blog.tipranks.com/fda-places-biomarins-gene-therapy-study-on-hold/
Published Date: Tue, 07 Sep 2021 15:32:03 +0000
Read More
Did you miss our previous article...
https://peaceofmindinvesting.com/investing/meridianlink-delivers-mixed-q2-results-shares-rise
.png) InvestingStocksToolsClubsVideosPrivacy PolicyTerms And Conditions
InvestingStocksToolsClubsVideosPrivacy PolicyTerms And Conditions
